RainbowSingularity
Valued Senior Member
im waiting to see if that sun spot erupts into an ejection.I would hazard
http://spaceweathergallery.com/indiv_upload.php?upload_id=152996
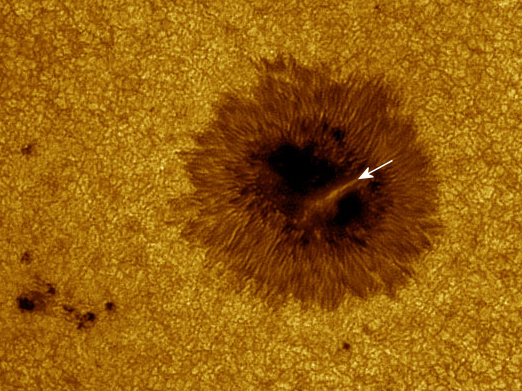
Does this mean sunspot AR2738 will explode--or quietly fall apart? No one can say. Readers with solar telescopes are encouraged to monitor developments.

