You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
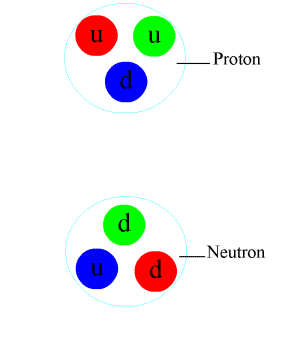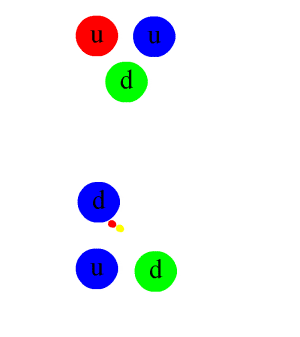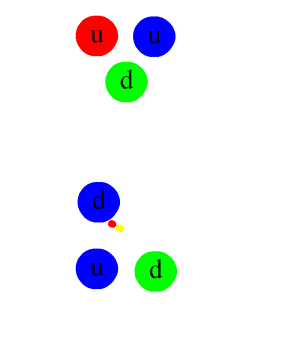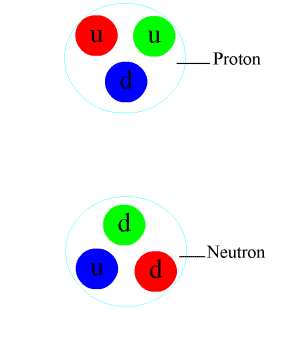
Positive charges in the nucleus
- Thread starter arauca
- Start date
It is call the strong nuclear force.What are the distances in the nucleus between protons , and what is holding them together so they are not break away from nucleus
Wikipedia covers it quite well at http://en.wikipedia.org/wiki/Strong_interaction
HistoryBefore the 1970s, physicists were uncertain about the binding mechanism of the atomic nucleus. It was known that the nucleus was composed of protons and neutrons and that protons possessed positive electric charge while neutrons were electrically neutral. However, these facts seemed to contradict one another. By physical understanding at that time, positive charges would repel one another and the nucleus should therefore fly apart. However, this was never observed. New physics was needed to explain this phenomenon.
A stronger attractive force was postulated to explain how the atomic nucleus was bound together despite the protons' mutual electromagnetic repulsion. This hypothesized force was called the strong force, which was believed to be a fundamental force that acted on the nucleons (the protons and neutrons that make up the nucleus). Experiments suggested that this force bound protons and neutrons together with equal strength.[citation needed]
It was later discovered that protons and neutrons were not fundamental particles, but were made up of constituent particles called quarks. The strong attraction between nucleons was the side-effect of a more fundamental force that bound the quarks together in the protons and neutrons. The theory of quantum chromodynamics explains that quarks carry what is called a color charge, although it has no relation to visible color.[1] Quarks with unlike color charge attract one another as a result of the strong interaction, which is mediated by particles called gluons.
It is call the strong nuclear force.
Wikipedia covers it quite well at http://en.wikipedia.org/wiki/Strong_interaction
That is nice , Then why neutron fall out from the nucleus and not the protons in case of radiation , does that means that the strong forces don't act upon the neutrons
That is nice , Then why neutron fall out from the nucleus and not the protons in case of radiation , does that means that the strong forces don't act upon the neutrons
It will have to do with they way they stack together. Some arrangements will have a lower energy level and be more stable than others. If it means it has to spit out a neutron to get there it will happen from time to time. They don't all react at once remember, radioactive half-lives etc.
It would be type of physical displacement I would say. Like squeezing a slippery orange pip and it flies off. The other neutrons/protons squeezing down on it eject it. Is that a good guess?In neutron emission, what force gives the emitted neutrons their 'kick' away from the nucleus?
Not EM or gravity, obviously.
So is it the weak nuclear force?
Or can the strong nuclear force be repulsive as well as attractive?
Meets the common sense test.
But it doesn't quite get to the answer.
In the macro world, physical displacement is about the electromagnetic force between atoms and molecules.
At this level, I think physical displacement could only be due to the Pauli exclusion principle.
If so, then what force does the Pauli exclusion principle use to keep apart two fermions which are attracted to each other?
Probably I'm asking the wrong question. I suspect I'm wrongly applying classical intuition to a quantum phenomenon.
But it doesn't quite get to the answer.
In the macro world, physical displacement is about the electromagnetic force between atoms and molecules.
At this level, I think physical displacement could only be due to the Pauli exclusion principle.
If so, then what force does the Pauli exclusion principle use to keep apart two fermions which are attracted to each other?
Probably I'm asking the wrong question. I suspect I'm wrongly applying classical intuition to a quantum phenomenon.
No you are on the right trackMeets the common sense test.
But it doesn't quite get to the answer.
In the macro world, physical displacement is about the electromagnetic force between atoms and molecules.
At this level, I think physical displacement could only be due to the Pauli exclusion principle.
If so, then what force does the Pauli exclusion principle use to keep apart two fermions which are attracted to each other?
Probably I'm asking the wrong question. I suspect I'm wrongly applying classical intuition to a quantum phenomenon.
Wiki on Pauli exclusion principle states
The consequence of the Pauli principle here is that electrons of the same spin are kept apart by a repulsive exchange interaction, which is a short-range effect, acting simultaneously with the long-range electrostatic or coulombic force. This effect is partly responsible for the everyday observation in the macroscopic world that two solid objects cannot be in the same place in the same time
When you try and fit two solid objects into the one place one has to fly off.
No you are on the right track
Wiki on Pauli exclusion principle states
When you try and fit two solid objects into the one place one has to fly off.
Thanks!
But I've gone too far... I just tried reading the Exchange interaction, and now I have to scrape my brains off the ceiling.
In neutron emission, what force gives the emitted neutrons their 'kick' away from the nucleus?
Not EM or gravity, obviously.
So is it the weak nuclear force?
Or can the strong nuclear force be repulsive as well as attractive?
I don't think it is some sort of repulsive force 'kicking'. My take is that the original nucleus is in some metastable state, and the separation of a neutron would be some sort of tunneling process to a lower energy state.
It would be type of physical displacement I would say. Like squeezing a slippery orange pip and it flies off. The other neutrons/protons squeezing down on it eject it. Is that a good guess?
But this does not explain why neutrons rather than protons are ejected. The Wiki page has nothing on it. There is a page for proton emission and it says proton emission does not occur in nature. Why neutrons?
Because the strong force is strongest between protons and neutrons.That is nice , Then why neutron fall out from the nucleus and not the protons in case of radiation , does that means that the strong forces don't act upon the neutrons
Oh, and there are two drip lines - only neutron rich (heavy) isotopes decay by emitting neutrons. Neutron poor (light) isotopes decay by electron capture, or proton emission.
Addendum:
Proton Emission on Wiki
...to date more than 25 isotopes have been found to exhibit proton emission. The study of proton emission has aided the understanding of nuclear deformation, masses and structure, and it is a wonderfully pure example of quantum tunneling...
My recollection is that beyond a certain distance, the strong force becomes repulsive.Or can the strong nuclear force be repulsive as well as attractive?
Addendum:
I got that the wrong way around. At distances <0.7fm it becomes repulsive. At 1 fm it is attractive, and begins to decay rapidly out to a distance of 2.5fm.