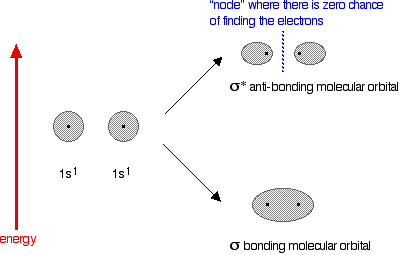
Thank you so much exchemist once again for doing your best. I've probably got a layout of the concept of particles as waves (thanks to your "light" example) but for now i think its better if i remember the basic concept of orbitals as "a region with a high probability of finding a an electron". Ill probably be studying QM sometime as well. I'm sure ill have a better idea of waves then. Ill be sure to look back to this essential thread as well. Thanks a lot
That is a question that is still argued over, among those interested in the philosophy of Quantum Theory. You get this with light of course. We can see light reflected, refracted and diffracted, and we know it has a wavelength and frequency, all of which are wave properties. But it seems it can only be emitted or absorbed in discrete units (which we call photons) and it can exert pressure on a surface on which it falls, which is particle-like behaviour. With beams of electrons and other particles of matter, we find we can also get diffraction effects (interference fringes for example). Which is wavelike behaviour! The wavelength of a particle is related to its momentum, by de Broglie's relation: lambda = h/p, in which lambda = wavelength, p = momentum and h is Planck's constant. So higher momentum particles have a shorter wavelength - or higher frequency.
As to what the "waves" are, that's a good question. The things that make a thing seem to exist are the properties of it that one can measure. With Quantum Mechanics one needs a short excursion into the maths and then back again to see how physical properties relate to the waves. In QM, all the information about a wave-particle in a particular state is contained in something called its "state function" or eigenfunction, or sometimes wavefunction. This complex-form mathematical function has wave-like qualities. (If you ever study alternating current theory in physics, you will find complex numbers crop up there as well - because that is also a theory of waves.) To determine the value of a physical property, such as energy or momentum, one "operates" mathematically on the state function with the appropriate QM operator. The simplest operation is for position. To find the probability of the particle being found in a given volume of space, one multiplies the function by its complex conjugate (this is the complex number equivalent of squaring it) and integrates this over the volume of space in question.
So, you can think of the "waves" as being waves of probability, or rather, the square root of probability. The pictures you see in textbooks of the shapes of orbitals are plots of the probability obtained in this way. But you cannot hope to know everything about a particle at once, in the way you can in classical physics. Heisenberg's Uncertainty Principle says that delta p.delta x >/=h/4 pi, in which delta p and delta x are the uncertainty about the momentum and position of the particle and h is again Planck's Constant. This is why you cannot track the "orbit" of an electron. If you could write down an orbit for it, you would be able to say what its position and momentum were exactly. In QM, this CANNOT be done. And the reason why is the inherently fuzzy nature of the probability waves which determine what you are allowed to know about a system of particles.
It is not easy and has troubled the best minds of science. Einstein himself hated it, saying God does not play dice. But, all the evidence is that God DOES play dice and that probability waves are how matter really does behave on the atomic scale.
My best advice to you, since you are starting out on this mysterious adventure, is to pay close attention to anything you are taught in physics concerning waves: wavelength and frequency, phase, amplitude and energy, reflection, refraction, diffraction, resonance and standing waves, constructive and destructive interference and what happens when you superimpose waves of different frequencies on top of one another. All these come up time after time in QM and are used to explain all sort of things in chemistry, from atomic and molecular spectra to chemical bonding itself. So if you understand waves, you will "get" QM - at least qualitatively.