DaveC426913
Valued Senior Member
About zero point one percent (0.1%).It does
How much energy does fission get , relative to the mass ?
About zero point one percent (0.1%).It does
How much energy does fission get , relative to the mass ?
About zero point one percent (0.1%).
Yup.Interesting
So in Nuclear power plants , we get 0.1 percent ( 0.1%) of the uranium energy potential ?
Yup.
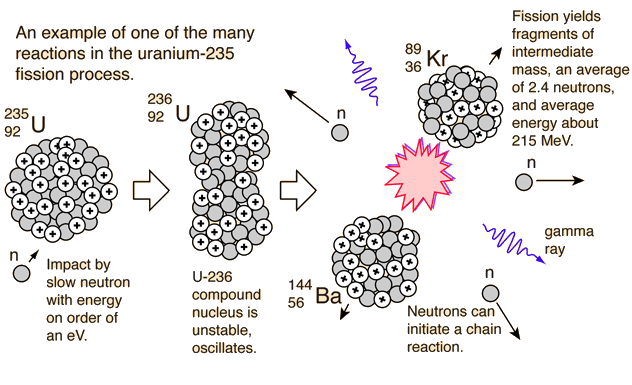
Fission is nowhere near annihilation. Uranium is split into (Krypton and Barium, I think) leaving 99.9% waste products.
The waste products have a combine mass juuuuuuust slightly less than that of Uranium. The missing mass has been converted to energetic particles.
If you want to know how much energy is released by a single uranium atom fissing, take the difference of the mass and multiply it by the speed of light squared.

(Krypton and Argon, I think)
I have no idea what you're talking about!Maybe Krypton and Barium?
Maybe Krypton and Barium?
Neither .

I was in the middle of a response to your other question about squaring c when this response came in.
This latest comment leads me to believe that you're not really interested in how this works. That you're going to re-assert your own ideas.
Is there any point in continuing? Or is this just about you?
What are the products of Uranium fission?Continue
What are the products of Uranium fission?
And the waste products?Alfa and beta particles .
And the waste products?
So, you don't think Uranium in a nuclear reactor fisses into Krypton and Barium?Nuclear decay radiation .
Lead , in the end .
So, you don't think Uranium in a nuclear reactor fisses into Krypton and Barium?
No, not directly. While Uranium fission results in the production of daughter isotopes which are radioactive and thus can decay via Alpha or Beta emission, the actual process of fission does not produce them.Alfa and beta particles .
No, not directly. While Uranium fission results in the production of daughter isotopes which are radioactive and thus can decay via Alpha or Beta emission, the actual process of fission does not produce them.
Then you are wrong. Fission is the process of a nucleus of a particular isotope breaking apart into to smaller parts which each make up a nucleus of a lighter element. The energy released comes from the fact that that the rest mass of the two daughter isotopes, plus that of any free neutrons produced, is less than the rest mass of the original nucleus.Not so far .
If you're not going to believe standard science, what is the point in my telling you about c^2?Not so far .
Then you are wrong. Fission is the process of a nucleus of a particular isotope breaking apart into to smaller parts which each make up a nucleus of a lighter element. The energy released comes from the fact that that the rest mass of the two daughter isotopes, plus that of any free neutrons produced, is less than the rest mass of the original nucleus.
