You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Absolute zero
- Thread starter Beaconator
- Start date
Write4U
Valued Senior Member
Reference to Dunning-Kruger even?No I did not. I stated my opinion of what using that sentence would imply about the user. But you have not used that sentence only asked if it could be used (to which the answer is obviously yes and also obviously listeners would be able to draw conclusions about the user and perhaps less obviously the user would probably not like those conclusions).
I parse my words very carefully. Apparently you are unable to follow the logic and look for "correct" language as if that implies understanding.I think you are not parsing your own sentences carefully so are reading things into my responses that are not there. Only if you are claiming the phrase in #13 as something you would say can there be any implication about you and you have not made such a claim.
OK, you have spit your venom. now slither away, please.
exchemist
Valued Senior Member
Let me try: In a body at absolute zero, the only energy remaining is zero point energy, i.e. the energy left in the ground state. Since there are no energy levels below the ground state, there can be no heat flow from it.Can one use the phrase: Absolute zero is the complete absence of heat-producing energy?
Write4U
Valued Senior Member
Thank you. That is what I was trying to say with the fewest words.Let me try: In a body at absolute zero, the only energy remaining is zero point energy, i.e. the energy left in the ground state. Since there are no energy levels below the ground state, there can be no heat flow from it.
Last edited:
DaveC426913
Valued Senior Member
W4u has been told this a dozen times by a dozen different members. Welcome to this not very exclusive club.But the "yes" is obvious. I simply pointed out a consequence that if you did use it then people would draw certain conclusions about the contrast between your apparent knowledge of physics and your apparent confidence in making declarative statements about physics.
exchemist
Valued Senior Member
Yeah, you just mangled the words, as you always do.Thank you. That is what I was trying to say with the fewest words.
In that case "absolute zero is the complete absence of heat". It's the "heat-producing energy" that makes what you originally wrote into nonsense.Thank you. That is what I was trying to say with the fewest words.
Write4U
Valued Senior Member
Now we are engaging in productive discourse.In that case "absolute zero is the complete absence of heat". It's the "heat-producing energy" that makes what you originally wrote into nonsense.
That short version was my first choice, but then I anticipated a question about what would cause the absence of heat and tried to use a shorthand version of exchemist's much lengthier explanation. Apparently, he saw fit to include the cause of lack of heat.
In a body at absolute zero, the only energy remaining is zero point energy
So now we have your even shorter version of mine which is somewhat vague, and a lengthier version that addresses the why and how the lack of heat.
I am beginning to think that my version might be a happy medium, addressing everything with the fewest words including a short-hand synopsis of the causal relationship.
Last edited:
It appears that what you originally asked ("can I use this") wasn't what you actually wanted to ask ("what is a better way of saying this"). Perhaps we'd get here quicker if you asked the actual question you wanted an answer to straight off.Now we are engaging in productive discourse.
No it isn't because "heat-producing energy" is nonsense. Heat is a form of energy it isn't produced by energy.I am beginning to think that my version might be a happy medium
Write4U
Valued Senior Member
Are there other forms of energy other than heat?It appears that what you originally asked ("can I use this") wasn't what you actually wanted to ask ("what is a better way of saying this"). Perhaps we'd get here quicker if you asked the actual question you wanted an answer to straight off.
No it isn't because "heat-producing energy" is nonsense. Heat is a form of energy it isn't produced by energy.
In a Flash

Light is a form of radiant energy.
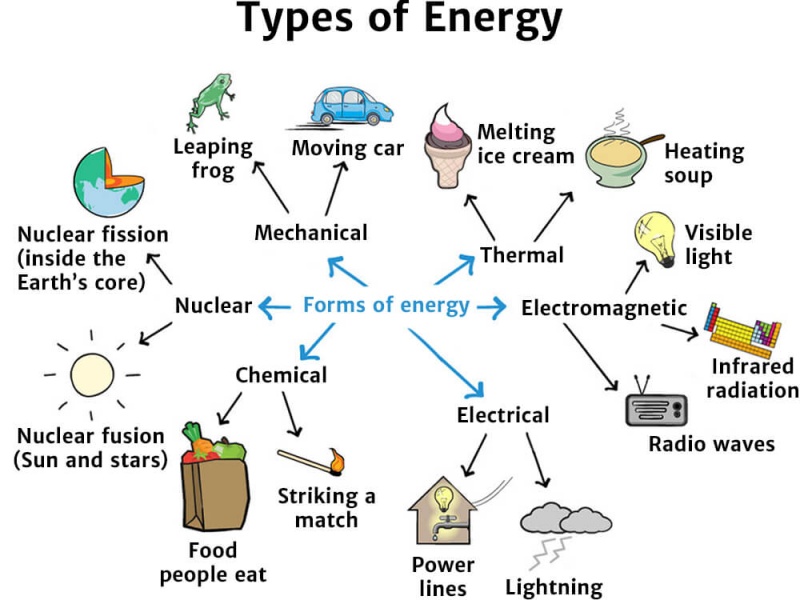
Forms of energy
There are many different types of energy, which all fall into two primary forms – kinetic and potential. Energy can transform from one type to another, but it can never be destroyed or created.
https://www.solarschools.net/knowledge-bank/energy/types
exchemist
Valued Senior Member
This area is a minefield, however, if there are pedants about.It appears that what you originally asked ("can I use this") wasn't what you actually wanted to ask ("what is a better way of saying this"). Perhaps we'd get here quicker if you asked the actual question you wanted an answer to straight off.
No it isn't because "heat-producing energy" is nonsense. Heat is a form of energy it isn't produced by energy.
 I have in the past been picked up by physicists for loose language, when talking of "heat energy", since strictly speaking it is internal energy flowing due to temperature difference.
I have in the past been picked up by physicists for loose language, when talking of "heat energy", since strictly speaking it is internal energy flowing due to temperature difference. For some reason this distinction was never really stressed during my education, with the result that if I'm not careful I think of heat as being the energy content of a body due to kinetic energy of its molecules. Which apparently is wrong.
It's correct for a monatomic gas which is the kind of ideal gas that's usually covered even up to early undergraduate courses but there's more that can go on with more general materials. The molecules of gases that have more than one atom can start vibrating or rotating when they collide which absorbs energy which it can later release to other materials when it collides with them. That's also heat. When you start talking about solids there are all of the vibrational modes of the solid called phonons that are also energy sinks.I think of heat as being the energy content of a body due to kinetic energy of its molecules. Which apparently is wrong.
In some senses you could call it all kinetic energy but it isn't all the simple translational kinetic energy of atoms or molecules moving around randomly that you might be imagining.
Last edited:
exchemist
Valued Senior Member
Yeah I meant k.e. in rotation and vibration too. (I did spectroscopy and some molecular QM at university).It's correct for a monatomic gas which is the kind of ideal gas that's usually covered even up to early undergraduate courses but there's more that can go on with more general materials. The molecules of gases that have more than one atom can start vibrating or rotating when they collide which absorbs energy which it can later release to other materials when it collides with them. That's also heat. When you start talking about solids there are all of the vibrational modes of the solid called phonons that are also energy sinks.
In some senses you could call it all kinetic energy but it isn't all the simple translational kinetic energy of atoms or molecules moving around randomly that you might be imagining.
My point was that heat seems to be a term used only for energy in transit, flowing from one body to another as a result of a temperature difference, not for the total internal energy present in all these degrees of freedom.
Some people say that but I bet they will also use terms like "heat reservoir" (a store of energy in transit that's not in transit) and "heat transfer" (the amount of energy in transit that transitted from one heat reservoir to another) so I wouldn't take such complaints too seriously.My point was that heat seems to be a term used only for energy in transit, flowing from one body to another as a result of a temperature difference, not for the total internal energy present in all these degrees of freedom.
exchemist
Valued Senior Member
Haha, you are probably right. But if I look up internet definitions, they indeed all seem to be confined to a flow of internal energy due to a temperature gradient.Some people say that but I bet they will also use terms like "heat reservoir" (a store of energy in transit that's not in transit) and "heat transfer" (the amount of energy in transit that transitted from one heat reservoir to another) so I wouldn't take such complaints too seriously.
Maybe some (other?
Write4U
Valued Senior Member
Here I am! The ignorant word mangler......
Methinks we just listened to a pair of pedants discussing the subtleties of thermal energy.
Thermal energy
Are all forms of energy heat?
Seems that not all energy IS heat!
Question: IS gravitational energy heat? Or does applied gravitational energy produce heat?
Question: in absolute zero, is gravity still effective?
Methinks we just listened to a pair of pedants discussing the subtleties of thermal energy.
Thermal energy
https://www.solarschools.net/knowledge-bank/energy/types/thermal#Thermal energy (also called heat energy) is produced when a rise in temperature causes atoms and molecules to move faster and collide with each other. The energy that comes from the temperature of the heated substance is called thermal energy.
Are all forms of energy heat?
https://vikaspedia.in/energy/energy-basics/forms-of-energyEnergy exists in many different forms. Examples of these are: light energy, heat energy, mechanical energy, gravitational energy, electrical energy, sound energy, chemical energy, nuclear or atomic energy and so on. Each form can be converted or changed into the other forms.
Seems that not all energy IS heat!
Question: IS gravitational energy heat? Or does applied gravitational energy produce heat?
Question: in absolute zero, is gravity still effective?
Last edited:
exchemist
Valued Senior Member
Of course all forms of energy are not heat. What a daft question.Here I am! The ignorant word mangler......
Methinks we just listened to a pair of pedants discussing the subtleties of thermal energy.
Thermal energy
https://www.solarschools.net/knowledge-bank/energy/types/thermal#
Are all forms of energy heat?
https://vikaspedia.in/energy/energy-basics/forms-of-energy
Seems that not all energy IS heat!
Question: IS gravitational energy heat? Or does applied gravitational energy produce heat?
Question: in absolute zero, is gravity still effective?
Write4U
Valued Senior Member
Reported for, "agreement, followed by ad hominem"Of course all forms of energy are not heat. What a daft question.
DaveC426913
Valued Senior Member
Reported for misusing terms the poster doesn't understand.Reported for, "agreement, followed by ad hominem"
